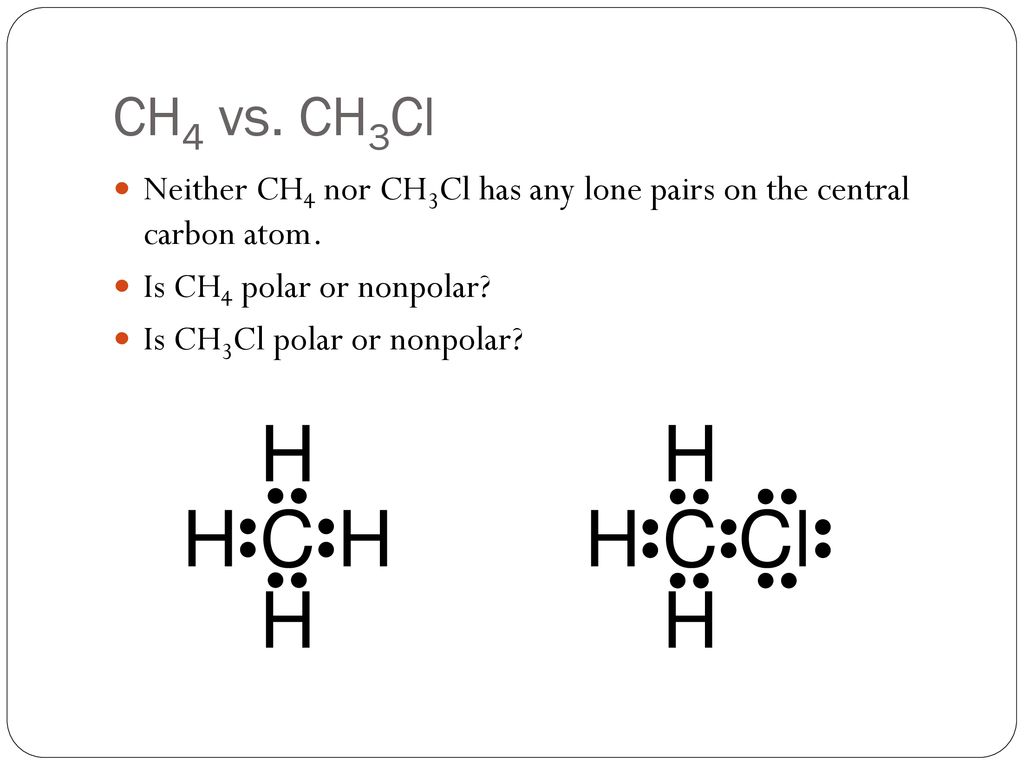
Explain Difference in Polarity in Ch4 and Ch3cl
The chlorine has a higher electronegativity than the hydrogen. Dipole moment of C.

What Is The Dipole Moment Of Ch4 Ccl4 Chcl3 H20 Nh3 In Urdu Hindi Class 11 Chemistry Youtube
The CH4 molecule two atoms are participating in bond formation Carbon and Hydrogen.

. The difference in electronegativity of the carbon and chlorine is 05. Posted April 9 2009 Kroughfire said. CH4 is a nonpolar molecule as it has a symmetric tetrahedral geometrical shape with four identical C-H bonds.
Kamladavi Dookie Pd 5 Question from video 1. The atoms have an electronegativity difference of 04 and both these atoms share a nonpolar bond. Which describe polarity of CH3Cl.
CH3F has dipole-dipole and London force intermolecular attractions whereas CH4 has only London force attractions. Methane is non-polar as the difference in electronegativities between carbon and hydrogen is not great enough to form a polarized chemical bond. This net dipole moment in the molecule makes Chloromethane a polar molecule.
Polarization of charge in the H-Cl bond is due to different electronegativities of chlorine and hydrogen. 1217 Explain in terms of intermolecular forces why a NH3 has a higher boiling point than CH4 and b KCl has a higher melting point than I2. 12K views View upvotes Related Answer Yashpreet Bathla.
Its high affinity for electron creates a dipole moment in C-Cl bond and hence net dipole is towards C-Cl making it polar molecule. The electronegativity of carbon and hydrogen is 255 and 22 respectively which causes the partial charges to be almost zero. By Joshua Mwanza Diamond.
Using Lewis structure we can infer that the C-Cl bond is polar and hence the CH3Cl is polar and has a net dipole. Since CH3Cl is bonded together stronger it will. In CH3Cl Cl has a different electronegativity than H so charges arent equally distributed.
I2 is a nonpolar molecular substance. Hence the molecule is polar compound. CH4 is symmetrical.
CH4 is tetrahedral in shape so each of the polarities of each of the C-H bonds cancel each other. This accounts for the high boiling points of these compounds. CH4 CH3CH3 CH2CH2 HC-CH.
CH3Cl is a fairly polar molecule. The charge distribution. CH3Cl because it is polar meaning it has both a dipole-dipole bond and dispersion bond where as CCl4 is non polar and only has a dispersion bond.
Since more the Cl more the dipole moment but in case of CH3Cl there is maximum dipole moment because ALL THE RESULTANTS ARE IN SAME DIRECTIONS. Cl is one of the more electronegative elements. Methane is non-polar because the carbon-hydrogen bonds have low polarity.
Therefore the molecule has net dipole. Which of the following concepts can be used to explain the difference in acidity of acetylene C2H2 and ethylene C2H4. There are no ion-ion ion-dipole or dipole-dipole forces in CH4 because those rely on the polarity of the molocule and because Cl is polar CHCl3 has more intermolecular forces and a higher boiling point.
Answer 1 of 7. Therefore the bond between carbon and chlorine is polar covalent. The structure for molecule is as follows.
However You have 3 C-H bond at tetrahedral angles but the C-Cl bond more polar than the C-H bond occupies the fourth apex of the tetrahedron and the CH3Cl is not canceled out as in. Both CH4 and CH3Cl has tetrahedral structure but CH4 is non polar while CH3Cl has polarity. Dipole-dipole and London dispersion forces exist between acetone molecules while 1-propanol has hydrogen bonding intermolecular forces.
BKCl is an ionic compound. The magnitude of the polarity of a bond is termed as the. 0 like 0 dislike.
The polarity of chloromethanes reveals the importance of symmetry. Because in CH4 the valence electrons of Carbon are being pulled away from the nucleus with the same force since all hydrogen has the same electronegativitySo charges are equally distributed throughout the atom and the atom is non-polar. Which of the following compounds are non-polar.
Its because Cl Chlorine is a very electronegative element. This will then reasult in charge distribution of CH3Cℓ being asymmetrical C-H and C-Cl bonds are of different strength and CH4 being symmetrical because of all same C-H bonds. CH3Cl is a polar molecule while CH4 is a nonpolar molecule because when you subtract the electronegativity values it will be between 20 and 05 while the electronegativity value difference of CH4 will be less than.
Therefore we do not draw any dipole arrow for C-H bonds. The ΔEN of carbon and hydrogen is 035 too weak to be considered a true polar bond. LDF relates to the size of the molocule giving CHCl3 more intermolecular forces than CH4already.
In case of CHCl3 resultant of TWO Cl bonds cancel each other and the remaining H and Cl gives the dipole moment. But if there is very small or no electronegativity difference between the bonded atoms the resulting bond is nonpolar in nature. Electronegativity plays a vital role in deciding whether the given molecule is polar or nonpolar.
If you calculate the difference 25-21 it comes to 04. The difference between electronegativity values of hydrogen and carbon is small and thus C-H bond is non-polar. CH3SH CH3OH CH3Cl CH3NH2.
The electronegativity of the Carbon atom is 25 and the electronegativity for Hydrogen atoms is 21. 1 CH3Cl has 3 C-H bonds and 1 C-Cl bond. IonIon forces are much stronger than any intermolecular forces.
I thought about it and I think it does have to do with ceCH4 is non polar so it does not tend to stick to each other gas state while ceCH3Cl is polar so it does stick such as like ceH2O which is liquid and is cohesive so ceCH3Cl would be as well. A CH3F has a polar CF bond whereas all the bonds in CH4 are of low polarity. Therefore CH4 has a lower boiling point than CH3F.
Here is CH3Cl as Chlorine has more electronegativity than all the other atoms it has a partial negative charge and the hydrogen atoms have partial positive charges. The difference in electrostatic potential is also minimal giving an overall nonpolar molecule. Methane CH 4 is a non-polar hydrocarbon compound composed out of a single carbon atom and 4 hydrogen atoms.
If the bonds were polar the molecule would still be non-polar becasue the four dipoles would cancel out due to the. Why does ch3f have a higher boiling point than ch4. Question 1Watch the video 1 and explain why CH3Cl is a polar molecule while CH4 is a nonpolar molecule.
It ha View the full answer. Is anyone able to tell me if I am on the right path. Symmetry is another factor responsible for the polarity of the bond.
The C-Cl bond has a large difference in electronegativity compared to the H-C bonds. Only weak dispersion forces are possible. Look up the 3D shape of each.
1-Propanol CH3CH2CH2OH has a molecular weight that is very similar to that of acetone yet acetone boils at 565 C and 1-propanol boils at 972 C. A bond is said to be polar if the bonded atoms have a considerable molecular bondelectronegativity difference between them. Which compound contains the most polar bond.
Since the three polar bonds are not symmetrical they do not cancel the dipole each other. CO2 NH3 H2O BCl3.
Polarity And Intermolecular Forces Ppt Download

Best Overview Is Chcl3 Polar Or Nonpolar Science Education And Tutorials

No comments for "Explain Difference in Polarity in Ch4 and Ch3cl"
Post a Comment